Search our Insights Archive
Purdie Pascoe’s Primary Market Research team
Our primary market research team combines deep expertise with a full range of qualitative and quantitative techniques, including the latest digital approaches with a wealth of experience across medical devices, digital healthcare, drug delivery, precision medicine and diagnostics.
The MedTech Clinical Network Conference
We are delighted to be silver sponsor for the MedTech Clinical Network Conference from 25-26 February in Brussels. Purdie Pascoe’s Post Market Survey experts Marcus Torr (Head of Post Market Surveys) Ellie Baker (Commercial Director) Chris Webb (Associate Director) and Matt Hanson (Senior Research Executive ) are exhibiting on Booth 1.
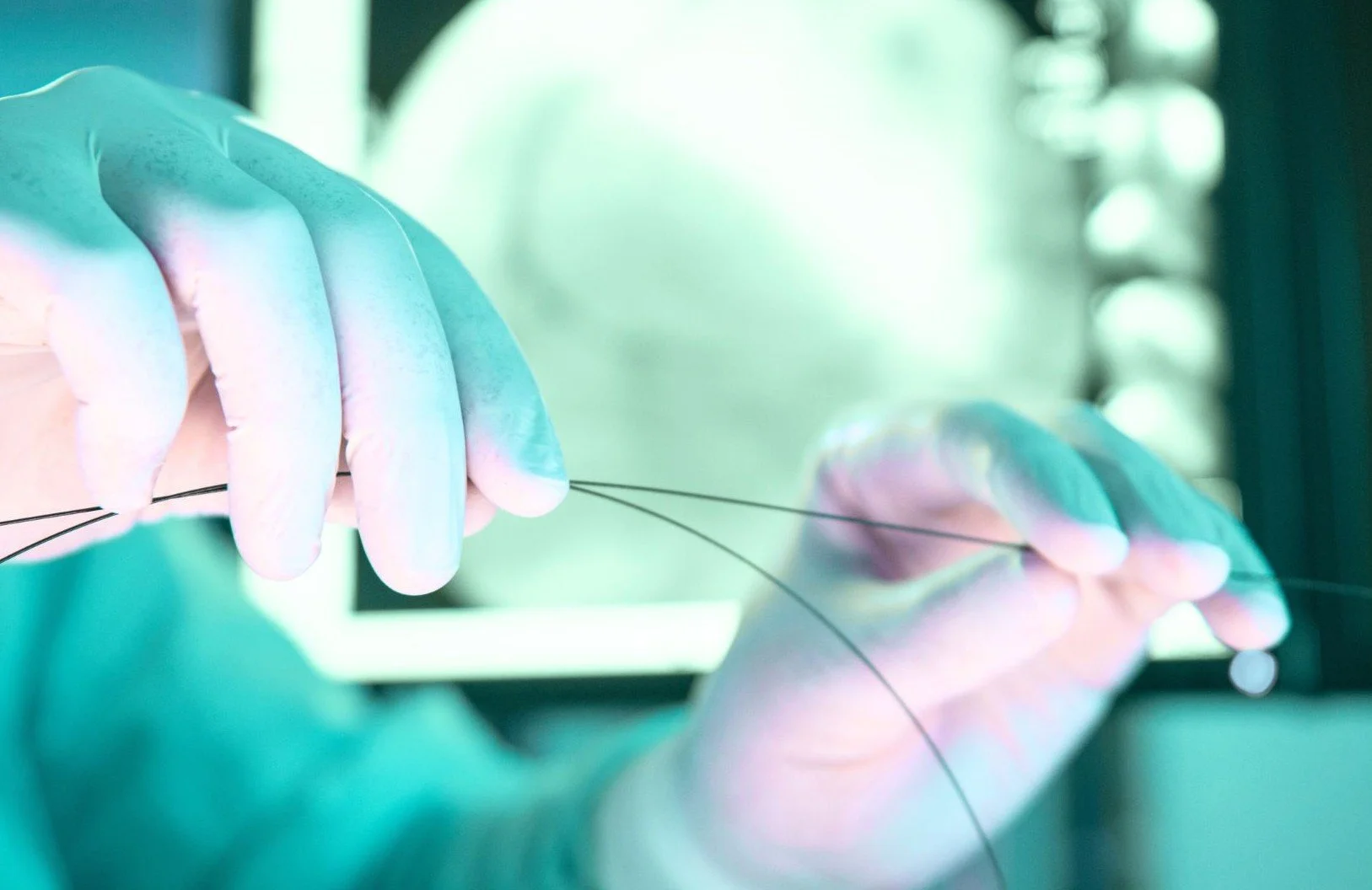
From data to confidence: PMCF survey success for a Class III Guidewire
As part of compliance with the EU Medical Device Regulation (MDR), the manufacturer of a Class III guidewire needed to generate robust Post Market Clinical Follow-Up (PMCF) data to confirm ongoing safety and performance. The study was designed to evaluate two key endpoints: procedural success, and freedom from adverse events.
The Global Shift in Real-World Evidence: Insights from MedTech Industry Leaders
This 1-hour live webinar on Wednesday, 11 February 2026 will bring together industry experts from Notified Body BSI, MDIC's NEST Initiative and specialists from Purdie Pascoe and 3Aware to unpack what these changes mean in practice and how MedTech manufacturers can build effective, compliant RWE strategies.
Smarter workflows, sharper insights: How our dedicated Innovation team helps us (and our clients) work better
Innovation sits right at the intersection of data, technology, and research. Our Innovation team’s goal is simple: make complex studies easier to run, insights easier to find, and decisions easier to make, for both our research colleagues and our clients.
Real-World Data: The fuel behind modern clinical evidence, and why Post Market Surveys are a smart way to capture it
As regulators worldwide including the FDA and EU Notified Bodies place greater emphasis on RWD, the industry faces a familiar but intensified challenge: how to generate high-quality, device-specific evidence efficiently, reliably, and without escalating costs. That’s where Post-Market Surveys come in.
Happy Holidays from Purdie Pascoe!
As we approach the end of 2025, the global Purdie Pascoe team wishes you a relaxing and fun holiday season with your loved ones. It has been a big year for us, from reaffirming our MedTech positioning and elevating our brand, to partnering with a range of existing and new clients across both our Primary Market Research and Post Market Survey teams. We’ve achieved all of this while having fun, staying true to our close-knit culture, and experiencing our biggest year of growth to date…
Movember in Motion: Internal study about how our people think about health and fitness
Have you ever wondered what really makes your employees tick when it comes to their health and wellbeing? What drives them to move, eat well, switch off mentally or what silently holds them back? Each November, millions join Movember to spark conversation and action around men’s physical and mental health. At Purdie Pascoe, we’ve taken part for the last few years. But this year, we wanted to go a step further. Instead of just looking outward, we turned the lens on ourselves.
Spotlight on a year in review at Purdie Pascoe
As we all start to get into the festive spirit ahead of the holiday season, it’s a perfect time to reflect on achievements, and of course challenges across the past year. 2025 has been a big year for Purdie Pascoe, and in this short interview, Co-Founder Guy Pascoe provides insight into what has made this year so pivotal for the business.
San Antonio Breast Cancer Symposium (SABCS) 2025
SABCS is one of the world’s most influential scientific meetings dedicated to breast cancer research and treatment. Are you attending? Meet Parisa Valadan, Associate Director to discuss how our team can help you clarify your strategy and uncover opportunities to accelerate impact.
Spotlight on why no longer just PMCF
Our dedicated division has evolved. While PMCF remains a core part of our offering, we’re now applying our expertise to broader areas, including other forms of Real World Data (RWD) collection and Post Market Performance Follow-Up (PMPF). In this conversation, Marcus and Ellie discuss the why behind the expansion of our services, the evolution of the team, and what it means for our clients at Purdie Pascoe.
Four rules for smarter ad testing in medical device marketing
Beneath the white coats, doctors are still human. They respond to good stories, authentic visuals, and messages that feel credible and relevant. At Purdie Pascoe, ad testing continues to be one of the most revealing parts of our work, helping medical device manufacturers understand not just what their audiences say they want, but what they actually respond to.
Reflections from MEDDEV Day and RAPS European Lifecycle Management Conference 2025
In this piece, Marcus Torr, Head of Post Market Surveys, shares his reflections on the discussions shaping the regulatory landscape, the themes emerging across both events, and how continued collaboration can drive meaningful improvements in patient safety and lifecycle management.
Are Multi Cancer Early Detection Tests (MCEDs) the new frontier in proactive healthcare for consumers?
Imagine a simple blood test that could screen for dozens of cancers, long before symptoms appear. This is the promise of Multi-Cancer Early Detection (MCED), a rapidly emerging field that has the potential to transform how we approach cancer screening globally. As with any breakthrough, the success of MCED will depend not only on the science, but also on public awareness, understanding and interest, healthcare system readiness, and equitable access.
Gender bias in medical device research: Why disaggregated data matters
By analysing data through a more granular lens, including disaggregation by gender, we can uncover meaningful insights, identify differential responses, and ultimately drive more equitable outcomes for all patient groups. Doing so also supports alignment with regulatory expectations around inclusive and evidence based post-market surveillance.
The MedTech Difference: Insights from Industry Leaders
Sabera Hyderally, Purdie Pascoe’s Head of US MedTech, recently facilitated an engaging, honest, and thought-provoking conversation with experts from across the MedTech and Diagnostics industry. The discussion explored how MedTech differs from other sectors, particularly pharmaceuticals, and what these differences mean for how we approach market research, innovation, and patient engagement.
Reflections from the 2025 RAPS Convergence: Putting Patients First and Embracing the Promise of AI
Attending this year’s RAPS Congress we were reminded of why we do what we do; at the heart of every conversation from regulatory reform to AI innovation there was one shared purpose: improving patients’ lives.
RAPS European Lifecycle Management Conference, Rotterdam
Are you attending the RAPS European Lifecycle Management Conference from 28-29 October? Purdie Pascoe’s Head of Post Market Surveys, Marcus Torr will be attending and presenting.
Purdie Pascoe global meeting 2025: MedTech, sunshine & the usual quiz night drama
With over 50 of us now based across the UK, US, and Europe, this year’s meeting was a true celebration of our growth, our people, and our commitment to MedTech. Fittingly, our theme this year was bold and simple: MedTech, as it’s not just one of our sectors - it’s our sole focus.
Spotlight on qualitative research in MedTech
While quantitative data can reveal what is happening, qualitative insights uncover the why; shedding light on patient experiences, clinician workflows, and unmet needs that numbers alone can't capture. By diving deep into real-world perspectives, MedTech companies can design more intuitive devices, craft compelling value propositions, and navigate complex regulatory and reimbursement landscapes with greater confidence.