Search our Insights Archive
The MedTech Clinical Network Conference
We are delighted to be silver sponsor for the MedTech Clinical Network Conference from 25-26 February in Brussels. Purdie Pascoe’s Post Market Survey experts Marcus Torr (Head of Post Market Surveys) Ellie Baker (Commercial Director) Chris Webb (Associate Director) and Matt Hanson (Senior Research Executive ) are exhibiting on Booth 1.
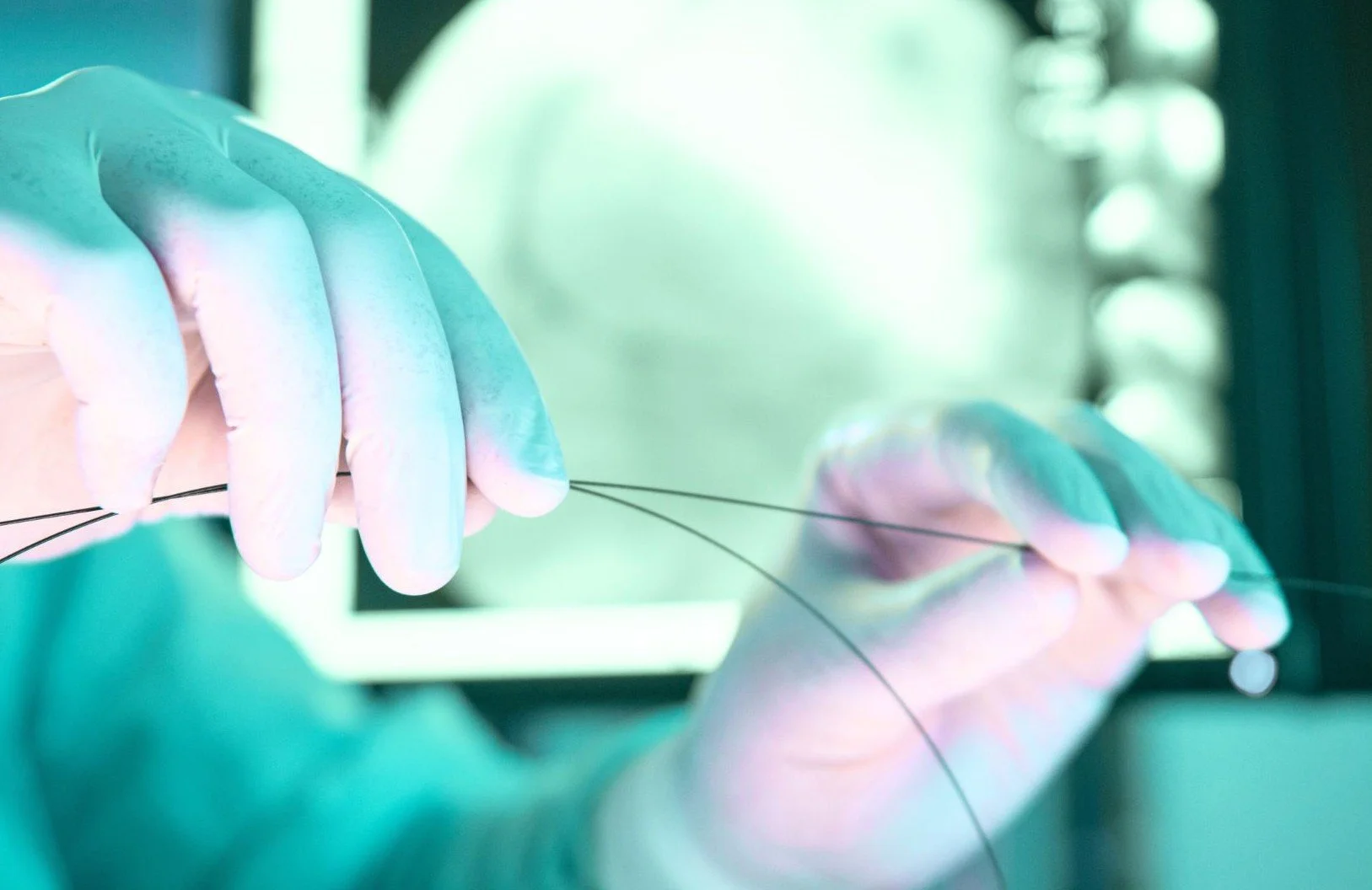
From data to confidence: PMCF survey success for a Class III Guidewire
As part of compliance with the EU Medical Device Regulation (MDR), the manufacturer of a Class III guidewire needed to generate robust Post Market Clinical Follow-Up (PMCF) data to confirm ongoing safety and performance. The study was designed to evaluate two key endpoints: procedural success, and freedom from adverse events.
The Global Shift in Real-World Evidence: Insights from MedTech Industry Leaders
This 1-hour live webinar on Wednesday, 11 February 2026 will bring together industry experts from Notified Body BSI, MDIC's NEST Initiative and specialists from Purdie Pascoe and 3Aware to unpack what these changes mean in practice and how MedTech manufacturers can build effective, compliant RWE strategies.
Real-World Data: The fuel behind modern clinical evidence, and why Post Market Surveys are a smart way to capture it
As regulators worldwide including the FDA and EU Notified Bodies place greater emphasis on RWD, the industry faces a familiar but intensified challenge: how to generate high-quality, device-specific evidence efficiently, reliably, and without escalating costs. That’s where Post-Market Surveys come in.
Reflections from MEDDEV Day and RAPS European Lifecycle Management Conference 2025
In this piece, Marcus Torr, Head of Post Market Surveys, shares his reflections on the discussions shaping the regulatory landscape, the themes emerging across both events, and how continued collaboration can drive meaningful improvements in patient safety and lifecycle management.
Gender bias in medical device research: Why disaggregated data matters
By analysing data through a more granular lens, including disaggregation by gender, we can uncover meaningful insights, identify differential responses, and ultimately drive more equitable outcomes for all patient groups. Doing so also supports alignment with regulatory expectations around inclusive and evidence based post-market surveillance.
RAPS European Lifecycle Management Conference, Rotterdam
Are you attending the RAPS European Lifecycle Management Conference from 28-29 October? Purdie Pascoe’s Head of Post Market Surveys, Marcus Torr will be attending and presenting.
How to Address Clinical Data Gaps Through Post-Market Clinical Follow-up Surveys
Post-Market Clinical Follow-up (PMCF) surveys can help address clinical data gaps that may impact the ongoing assessment of a medical device’s safety and performance. Unearthing clinical gaps requires a systematic approach, including a review of clinical literature, post-market surveillance data, and regulatory compliance standards.
MedDev Day, Budapest
We are delighted to be attending MedDev Day from 13-14 October 2025 in Budapest. Purdie Pascoe’s Head of Post Market Surveys, Marcus Torr will be attending and presenting at the conference.
RAPS Convergence, US
We are delighted to be the exhibiting at the RAPS Convergence, 7-9 October 2025 in Pittsburgh, US. Purdie Pascoe’s Post Market Survey experts Ellie Baker, Commercial Director and Chris Webb Research Manager | RCC- MDR are exhibiting on Booth 817.
Medical devices in the wild: Tracking real world data for lasting impact
Experts presenters from across the industry; Breda Kearny - Clinical Regulatory Lead, BSI, Diane M. Legere - Senior Clinical Auditor, DNV, Marcus Torr - Head of Post Market Surveys, Purdie Pascoe and Efsthathios Vassiliadis - CEO, Evnia.
Article 61(10) within the EU MDR | Notified Body Insights & the Role of PMCF Surveys
In this session, Breda Kearney, Clinical Regulatory Lead from BSI and Chris Webb, Research Manager and PMCF expert from Purdie Pascoe explore the correct application of Article 61(10) under the EU MDR and the critical role of PMCF surveys in supporting compliance.
Spotlight on Data and Quality
Harry Thomas is our dedicated Data Quality Assurance Officer at Purdie Pascoe. In this interview, Harry shares insights about his role and key processes from handling discrepancies or anomalies in collected data to how to adapt when working with new or evolving data collection technologies.
How we helped our client expand the indication of their device through a survey collecting real world data
Our client’s device is used for a specific indication, however users identified a potential benefit of the device’s design to perform for another indication. This study differs from typical PMCF efforts, with an aim to update the device’s labelling (IFUs) with safety and feasibility data for the expanded use. Find out how we heled out client in collecting retrospective real-world data to support the indication expansion.
Spotlight on MedTech Commercial Strategy
Ellie Baker recently joined Purdie Pascoe as PMCF Commercial Director. In this interview, Ellie shares why medical device manufacturers consider PMCF surveys, how she helps guide clients through the complexities of the MedTech landscape and how her first few weeks have been at the business.
Insights from the RAPS Euro Convergence in Brussels
The Purdie Pascoe Post Market Clinical Follow-up (PMCF) team was out in full force at this this year’s Regulatory Affairs Professionals Society (RAPS) convergence in Brussels. Marcus Torr, PMCF/MDR Lead, attended with newly appointed PMCF Commercial Director, Ellie Baker and PMCF Managers, Chris Webb and Tonika Chester.
Spotlight on Medical Writing
As Senior PMCF Medical Writer at Purdie Pascoe, Priyal Sharma offers her perspective about the benefits of having an in-house medical writer at an insights agency, how she ensures accuracy and clarity when communicating complex medical information and how she collaborates with the wider PMCF team.
Ellie Baker joins Purdie Pascoe as PMCF Commercial Director
We are delighted to announce that Ellie Baker has joined Purdie Pascoe’s Post Market Clinical Follow-Up Survey division as Commercial Director. Ellie brings over a decade of experience in healthcare market research, strategy, and business development, with a strong focus on MedTech and Pharma.
RAPS Euro Convergence 2025
We are delighted to be the exhibiting and presenting at the RAPS Euro Convergence, 13-16 May 2025 in Brussels. Purdie Pascoe’s PMCF experts, Marcus Torr, Chris Webb and Tonika Chester are attending, presenting a poster and exhibiting on Booth 37.
Insights from the TT Lifesciences Clinical Evidence Conference
PMCF experts Chris Webb and Alice Robertson recently attended and presented at the 6th Annual European Medical Device and Diagnostic Clinical Evidence Strategy Conference in Berlin. This event brought together leading clinical experts and industry professionals to discuss key issues in advancing clinical evidence.