Search our Insights Archive
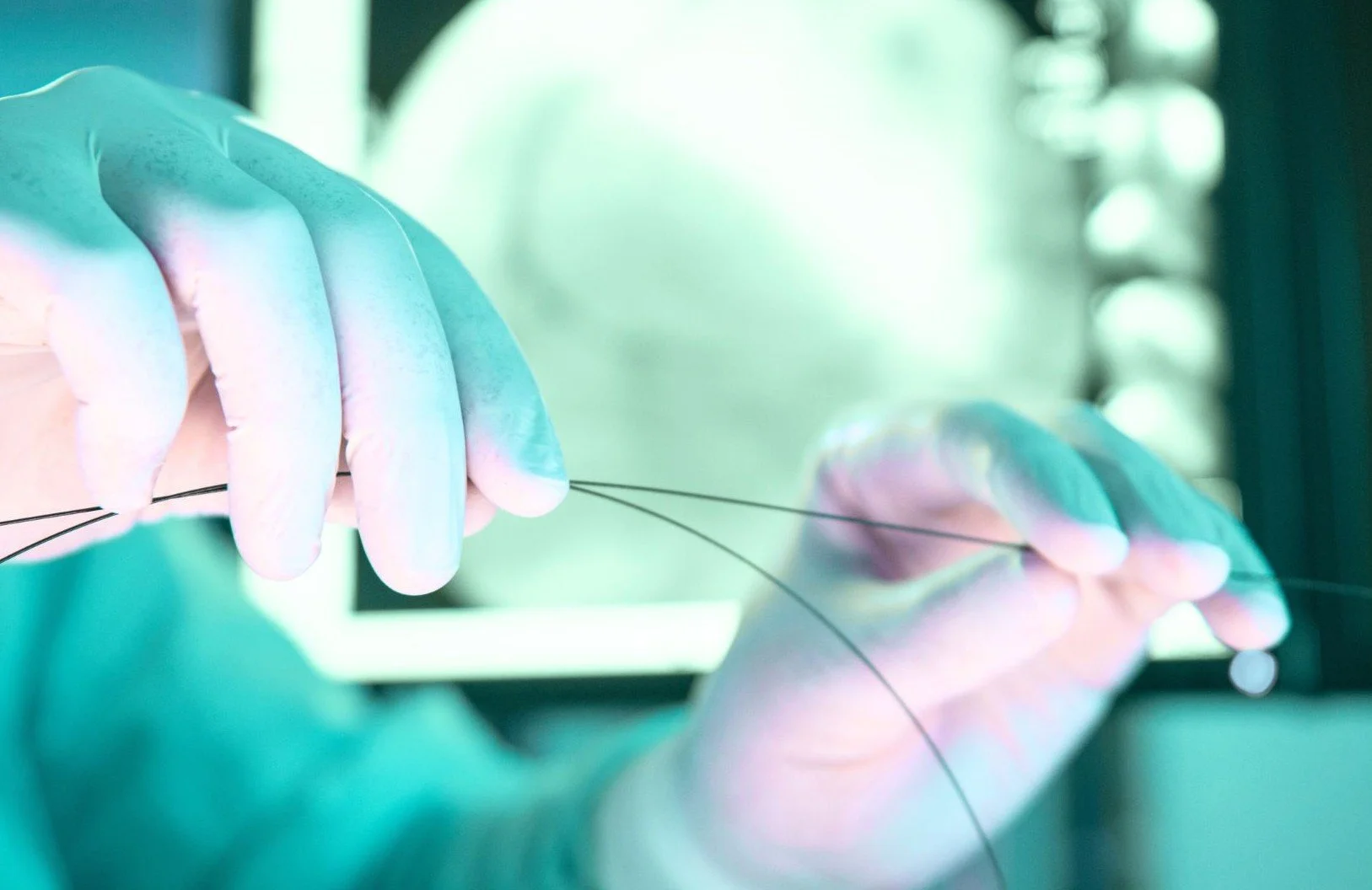
From data to confidence: PMCF survey success for a Class III Guidewire
As part of compliance with the EU Medical Device Regulation (MDR), the manufacturer of a Class III guidewire needed to generate robust Post Market Clinical Follow-Up (PMCF) data to confirm ongoing safety and performance. The study was designed to evaluate two key endpoints: procedural success, and freedom from adverse events.
Real-World Data: The fuel behind modern clinical evidence, and why Post Market Surveys are a smart way to capture it
As regulators worldwide including the FDA and EU Notified Bodies place greater emphasis on RWD, the industry faces a familiar but intensified challenge: how to generate high-quality, device-specific evidence efficiently, reliably, and without escalating costs. That’s where Post-Market Surveys come in.
Spotlight on a year in review at Purdie Pascoe
As we all start to get into the festive spirit ahead of the holiday season, it’s a perfect time to reflect on achievements, and of course challenges across the past year. 2025 has been a big year for Purdie Pascoe, and in this short interview, Co-Founder Guy Pascoe provides insight into what has made this year so pivotal for the business.
Spotlight on why no longer just PMCF
Our dedicated division has evolved. While PMCF remains a core part of our offering, we’re now applying our expertise to broader areas, including other forms of Real World Data (RWD) collection and Post Market Performance Follow-Up (PMPF). In this conversation, Marcus and Ellie discuss the why behind the expansion of our services, the evolution of the team, and what it means for our clients at Purdie Pascoe.
Reflections from MEDDEV Day and RAPS European Lifecycle Management Conference 2025
In this piece, Marcus Torr, Head of Post Market Surveys, shares his reflections on the discussions shaping the regulatory landscape, the themes emerging across both events, and how continued collaboration can drive meaningful improvements in patient safety and lifecycle management.
Gender bias in medical device research: Why disaggregated data matters
By analysing data through a more granular lens, including disaggregation by gender, we can uncover meaningful insights, identify differential responses, and ultimately drive more equitable outcomes for all patient groups. Doing so also supports alignment with regulatory expectations around inclusive and evidence based post-market surveillance.
Reflections from the 2025 RAPS Convergence: Putting Patients First and Embracing the Promise of AI
Attending this year’s RAPS Congress we were reminded of why we do what we do; at the heart of every conversation from regulatory reform to AI innovation there was one shared purpose: improving patients’ lives.
Purdie Pascoe global meeting 2025: MedTech, sunshine & the usual quiz night drama
With over 50 of us now based across the UK, US, and Europe, this year’s meeting was a true celebration of our growth, our people, and our commitment to MedTech. Fittingly, our theme this year was bold and simple: MedTech, as it’s not just one of our sectors - it’s our sole focus.
Spotlight on qualitative research in MedTech
While quantitative data can reveal what is happening, qualitative insights uncover the why; shedding light on patient experiences, clinician workflows, and unmet needs that numbers alone can't capture. By diving deep into real-world perspectives, MedTech companies can design more intuitive devices, craft compelling value propositions, and navigate complex regulatory and reimbursement landscapes with greater confidence.
Medical devices in the wild: Tracking real world data for lasting impact
Experts presenters from across the industry; Breda Kearny - Clinical Regulatory Lead, BSI, Diane M. Legere - Senior Clinical Auditor, DNV, Marcus Torr - Head of Post Market Surveys, Purdie Pascoe and Efsthathios Vassiliadis - CEO, Evnia.
Post Market Surveys: Insights from the poolside
As MedTech professionals know, the industry rarely sleeps, but let’s be honest: in August, it definitely takes a nap. With inboxes a little quieter and decision-makers (hopefully) stealing a few hours in the sun, it’s the perfect time for some lighter reflection. So, grab your iced coffee (or something stronger), and let’s talk Post Market Surveys, from the poolside.
Spotlight on MedTech end-to-end solutions
Partnering with an external team that understands and supports every stage of the MedTech journey can be transformative. In this conversation, leading experts share how Purdie Pascoe’s integrated expertise in Primary Market Research and Post Market Surveys is the true golden ticket to a seamless, compliant, and impactful approach for success in your MedTech journey.
A message from co-founders Marianne Purdie and Guy Pascoe
In a world where many agencies claim to serve MedTech, very few are true specialists. MedTech isn’t just one of our sectors - it’s our sole focus. That’s what sets us apart. We’re proudly returning to our roots and doubling down on what we do best; helping companies like yours make smarter, faster, and more confident decisions.